Tumor-specific Antigen (TSA) Targeted Therapy Utilizing Both NEO-102 and NEO-201 Clinical Programs
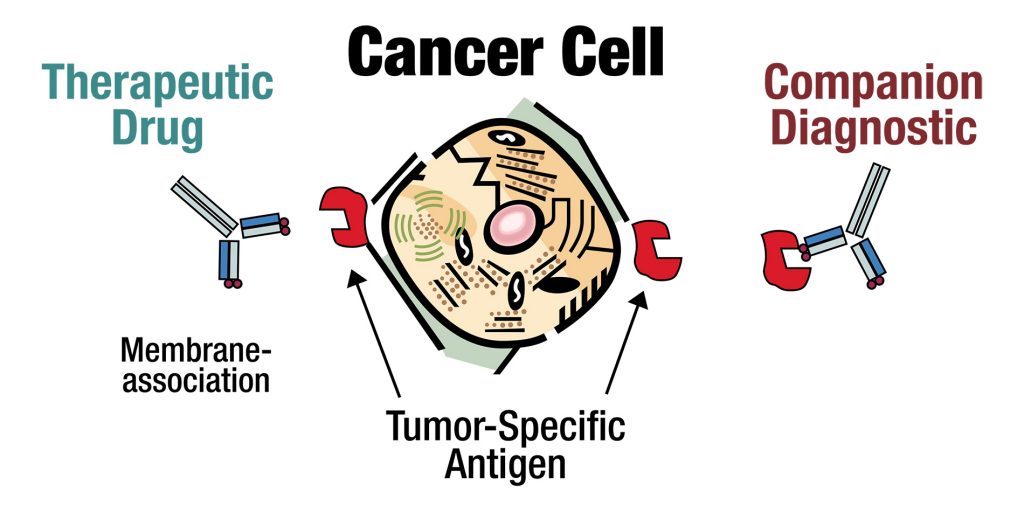
- Tumor-specific antigen detected by companion diagnostic
- Companion diagnostic currently used to pre-select therapy- responsive patients
- Precision Biologics has developed an assay using our current antibodies to stain cancer tissue (immunohistochemistry or IHC) from patients to determine if the target for our therapeutic antibody is present.
- This assay allows us to predict which patients have the best chance to respond to our antibody therapy
- Precision Biologics has completed its NEO-102 IHC Validation Report which was submitted to the US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH)
- Precision Biologics in conjunction with the Laboratory of Pathology at the National Cancer Institute has developed a Validated and Qualified NEO-201 IHC diagnostic assay
- The NEO-201 IHC assay is currently being employed to screen subjects for the ongoing Phase 2b clinical trial at the NCI in patients with metastatic 2nd line HNSCC, NSCLC, endometrial cancer and cervical cancer
- These assays may also have additional utilities as a diagnostic test, including early detection of cancer
- Other assays using patients’ blood are being developed with Precision Biologics antibodies including a serum ELISA test that may have additional utility to monitor patients undergoing therapy for cancer
