Our History
These ambitious efforts are rooted in the work of Dr. Myron Arlen. An esteemed surgical oncologist at Memorial Sloan-Kettering and later North Shore University Hospital, Dr. Arlen collaborated in the 1970s and 1980s with Dr. Ariel Hollinshead as Principal Investigator (PI) for particular clinical trials of Hollinshead cancer vaccines. Dr. Hollinshead, a pioneer in immunotherapy, worked with many types of cancer to isolate a variety of tumor-associated antigens (TAAs) from the tumors of numerous cancer patients. These TAAs were administered to cancer patients in the United States and many other countries around the world during the late 1970s and early 1980s in Phase I, II and III clinical trials.
The data from some of those trials suggested anti-tumor responses and prolonged survival for patients with cancer, with no significant adverse effects. However, at the time of these clinical trials, genetic engineering technology was in its infancy and it wasn’t possible to identify the active components of the TAAs or vaccines. In fact, it was not until very recent years that this vaccine library could be thoroughly explored at the molecular level.
As break throughs in biotechnology emerged, Dr. Arlen and several associates understood the great potential in the human-tested vaccines that Dr. Hollinshead still possessed. In 2004, Neogenix Oncology, Inc. obtained sole ownership of the Hollinshead library of vaccines, antibodies and antigens. In September 2012, Precision Biologics, Inc. purchased the library of vaccines, antibodies, antigens, and other assets from Neogenix Oncology, Inc.
The mission of Precision Biologics is to improve and extend the lives of cancer patients by developing innovative diagnostics and therapeutics that target tumors with precision.
OUR HISTORY
Company founded
Precision Biologics, Inc
Clinical Trials
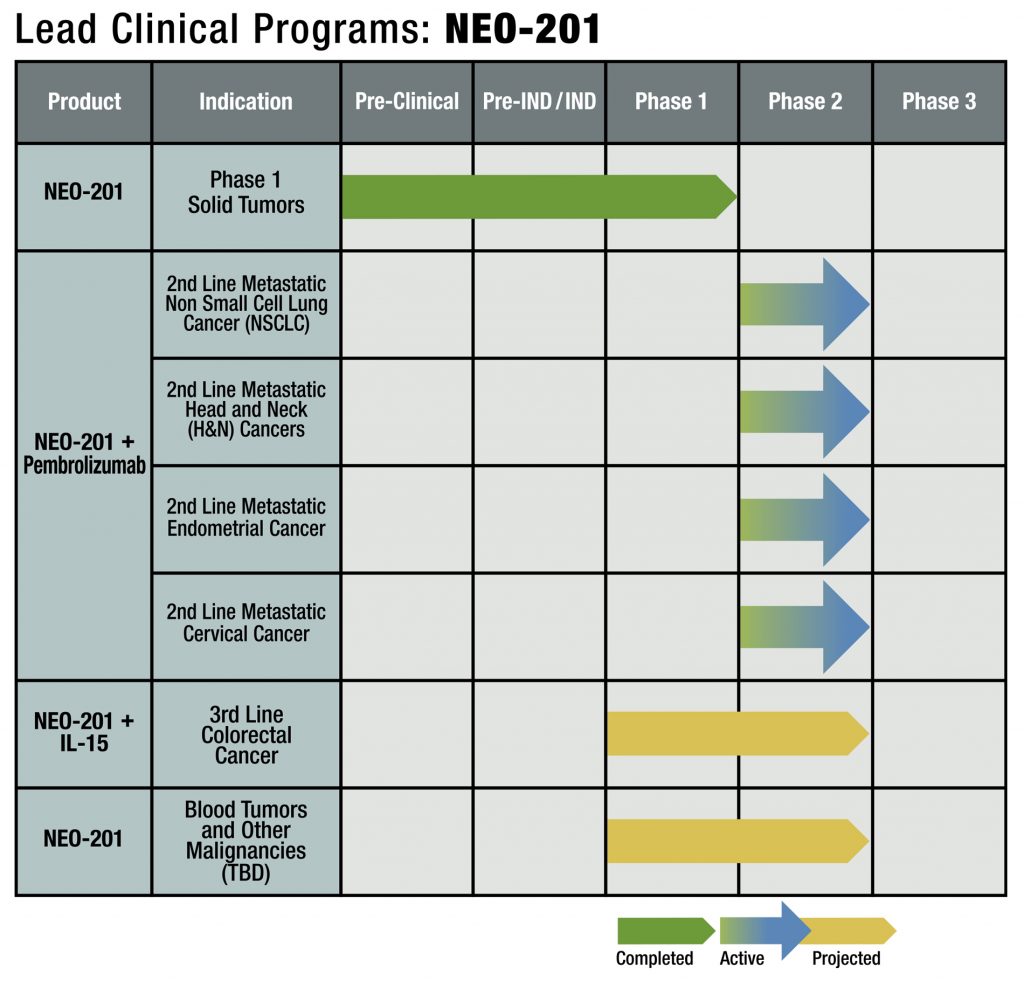
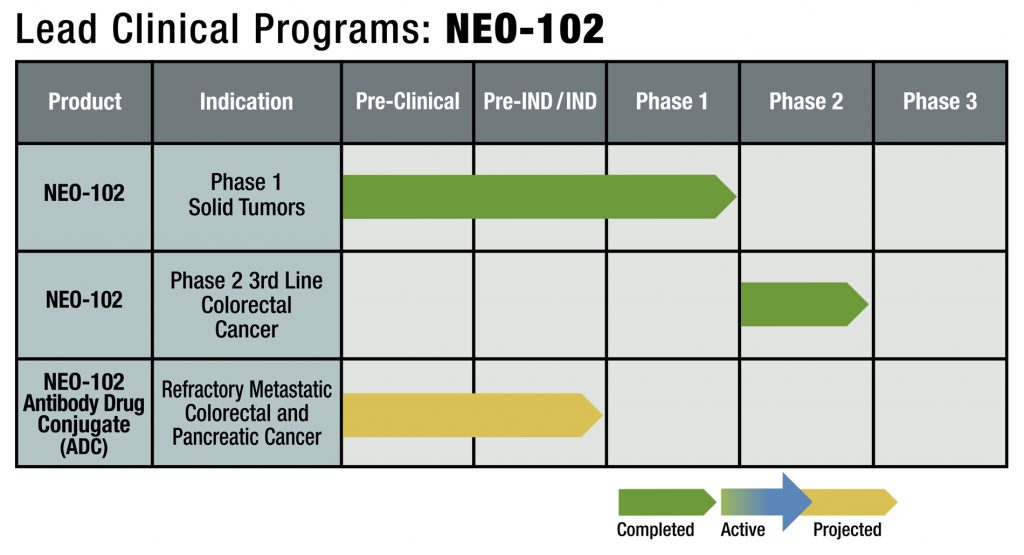
2nd monoclonal antibody NEO-201 commenced Phase I clinical trial
Development of companion diagnostic NEO-201 IHC
Initiation of NEO-201 Phase 2 combination Clinical Trial

Meet our Team




